We are focused on developing, co-developing, in-licensing, manufacturing, marketing, distribution and selling of innovative pharmaceutical products Viz bioequivalent generics, Value added generics (VAG’s), 505(b)(2), Para IV patent challenges, Niche therapeutics and NDDS dosage forms. The company operates through its own development and manufacturing hub in India and Co-development/in-licensing partnerships across the globe.


“To care for the well-being and happiness of the people.”


Creekwood Pharmaceuticals, LLC is engaged in development, co- development, in licensing, manufacturing, distribution,sales and marketing of innovative pharmaceutical products, designed to help patients with serious, acute or chronic conditions to better manage their disease resulting in overall well-being and happiness.

The five core values which guide our organization.

We work with uncompromising honesty and integrity in what we do and how we do.

We value people and the environment around us. We treat people with dignity and respect. We are compassionate towards people & the environment..

Competitive advantage through innovation.

Relentless focus on customers by servicing customer needs and ensuring we are responsive to customer demands, thereby creating a dedicated customer base.

We work with collaborative teamwork and encourage each other to achieve challenging goals.

| NDC | 82619-131-02 |
| GTIN | '00382619131024 |
| Strength | 200 mg |
| Description of Pill | Hydroxychloroquine sulfate tablets, USP 200 mg is white, capsule shaped film-coated tablets debossed with "ꓲꓲꓲ" on one side and plain on other side. |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | PLAQUENIL |
| Pack Size | 500 |
| Therapeutic Class | Antimalarial and Antirheumatic |

| NDC | 82619-131-01 |
| GTIN | 00382619131017 |
| Strength | 200 mg |
| Description of Pill | Hydroxychloroquine sulfate tablets, USP 200 mg is white, capsule shaped film-coated tablets debossed with "ꓲꓲꓲ" on one side and plain on other side. |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | PLAQUENIL |
| Pack Size | 100 |
| Therapeutic Class | Antimalarial and Antirheumatic |
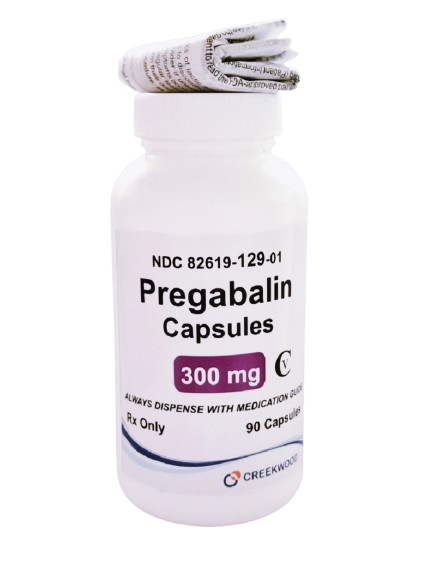
| NDC | 82619-129-01 |
| GTIN | 00382619129014 |
| Strength | 300 mg |
| Description of Pill | Pregabalin 300 mg capsules are"Size 1" White body and Orange cap, hard-gelatin capsule printed with black ink "CW" on the cap, and "300" on the body containing white to off- white crystalline powder. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Lyrica |
| Pack Size | 90 |
| Therapeutic Class | Anticonvulsant |
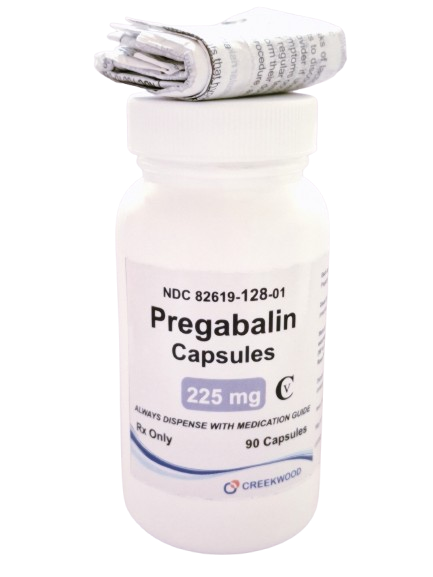
| NDC | 82619-128-01 |
| GTIN | 00382619128017 |
| Strength | 225 mg |
| Description of Pill | Pregabalin 225 mg capsules are"Size 1" White body and Light Orange, hard-gelatin capsule printed with black ink "CW" on the cap, and "225" on the body containing white to off- white crystalline powder. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Lyrica |
| Pack Size | 90 |
| Therapeutic Class | Anticonvulsant |
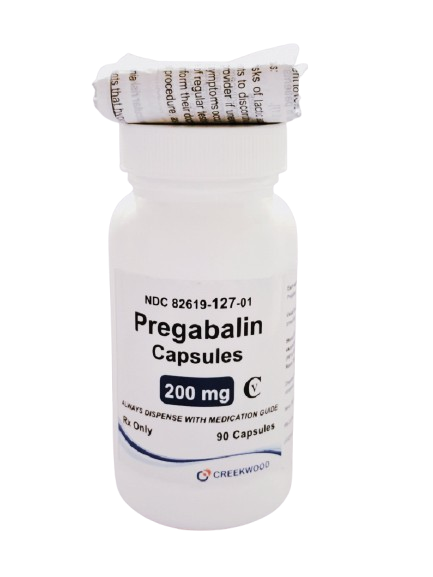
| NDC | 82619-127-01 |
| GTIN | 00382619127010 |
| Strength | 200 mg |
| Description of Pill | Pregabalin 200mg capsules are"Size 1" Light Orange, hard-gelatin capsule printed with black ink "CW" on the cap, and "200" on the body containing white to off- white crystalline powder. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Lyrica |
| Pack Size | 90 |
| Therapeutic Class | Anticonvulsant |
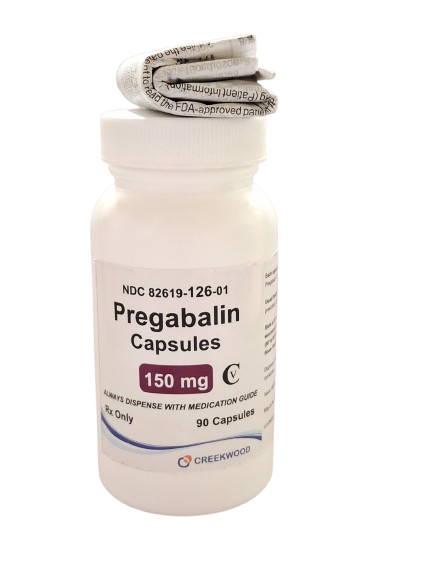
| NDC | 82619-126-01 |
| GTIN | 00382619126013 |
| Strength | 150 mg |
| Description of Pill | Pregabalin 150mg capsules are"Size 2" White, hard-gelatin capsule printed with black ink "CW" on the cap, and "150" on the body containing white to off- white crystalline powder. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Lyrica |
| Pack Size | 90 |
| Therapeutic Class | Anticonvulsant |
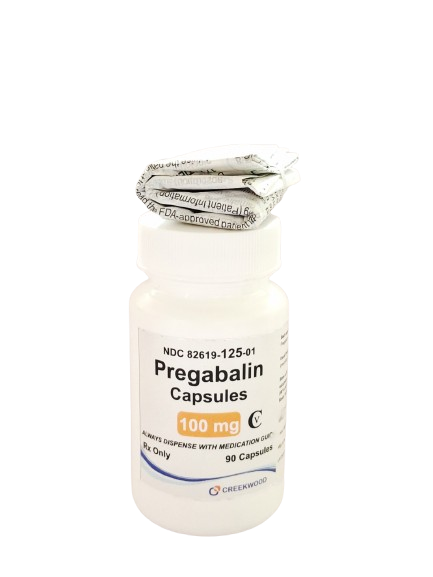
| NDC | 82619-125-01 |
| GTIN | 00382619125016 |
| Strength | 100 mg |
| Description of Pill | Pregabalin 100mg capsules are"Size 3" Orange, hard-gelatin capsule printed with black ink "CW" on the cap, and "100" on the body containing white to off- white crystalline powder. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Lyrica |
| Pack Size | 90 |
| Therapeutic Class | Anticonvulsant |
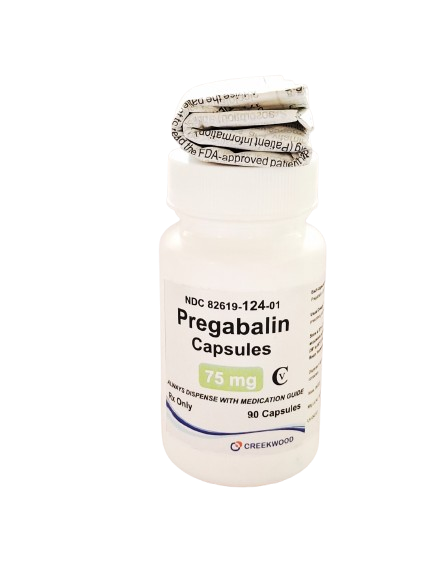
| NDC | 82619-123-01 |
| GTIN | 00382619124019 |
| Strength | 75 mg |
| Description of Pill | Pregabalin 75mg capsules are"Size 4" White body and orange cap, hard-gelatin capsule printed with black ink "CW" on the cap, and "75" on the body containing white to off- white crystalline powder. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Lyrica |
| Pack Size | 90 |
| Therapeutic Class | Anticonvulsant |
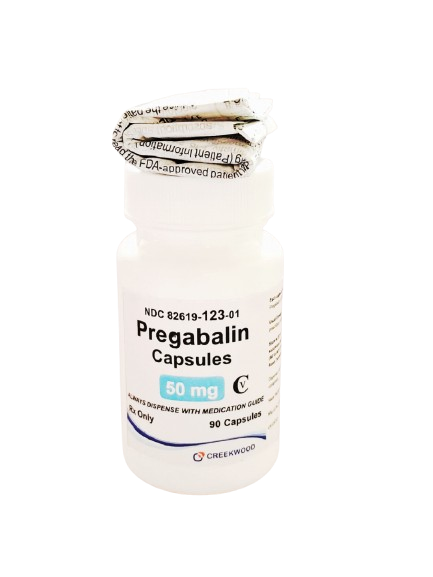
| NDC | 82619-123-01 |
| GTIN | 00382619123012 |
| Strength | 50 mg |
| Description of Pill | Pregabalin 50mg capsules are"Size 3" White, hard-gelatin capsule printed with black ink "CW" on the cap, and "50" on the body containing white to off white crystalline powder. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Lyrica |
| Pack Size | 90 |
| Therapeutic Class | Anticonvulsant |
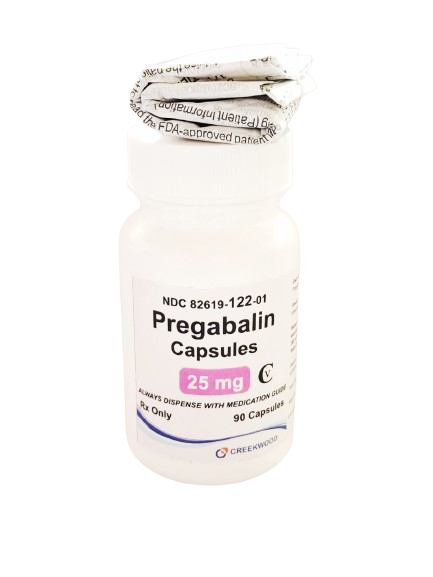
| NDC | 82619-122-01 |
| GTIN | 00382619122015 |
| Strength | 25 mg |
| Description of Pill | Pregabalin 25mg capsules are"Size 4" White, hard-gelatin capsule printed with black ink "CW" on the cap, and "25" on the body containing white to off white crystalline powder |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Lyrica |
| Pack Size | 90 |
| Therapeutic Class | Anticonvulsant |

| NDC | 82619-146-02 |
| GTIN | 00382619146028 |
| Strength | 800 mg |
| Description of Pill | White to off white, Modified Capsule Shape, biconvex film coated tablet debossed SG on one side and 178 on other side with bisect line on both sides. |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 500 |
| Therapeutic Class | Anticonvulsant |

| NDC | 82619-146-01 |
| GTIN | 00382619146011 |
| Strength | 800 mg |
| Description of Pill | White to off white, Modified Capsule Shape, biconvex film coated tablet debossed SG on one side and 178 on other side with bisect line on both sides. |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 100 |
| Therapeutic Class | Anticonvulsant |
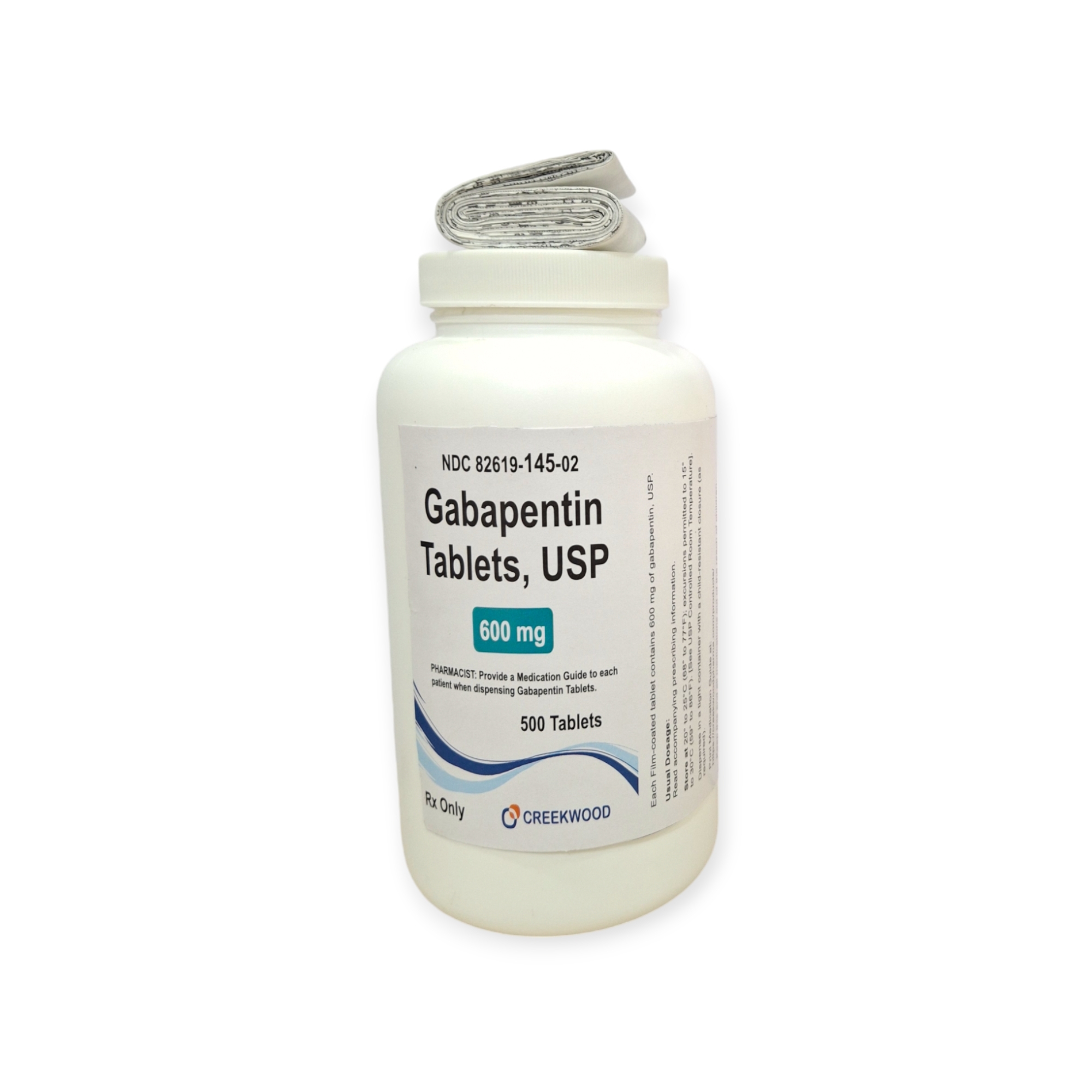
| NDC | 82619-145-02 |
| GTIN | 00382619145021 |
| Strength | 600 mg |
| Description of Pill | White to off white, Modified Capsule Shape, biconvex film coated tablet debossed SG on one side and 177 on other side with bisect line on both sides |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 500 |
| Therapeutic Class | Anticonvulsant |

| NDC | 82619-145-01 |
| GTIN | 00382619145014 |
| Strength | 600 mg |
| Description of Pill | White to off white, Modified Capsule Shape, biconvex film coated tablet debossed SG on one side and 177 on other side with bisect line on both sides |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 100 |
| Therapeutic Class | Anticonvulsant |
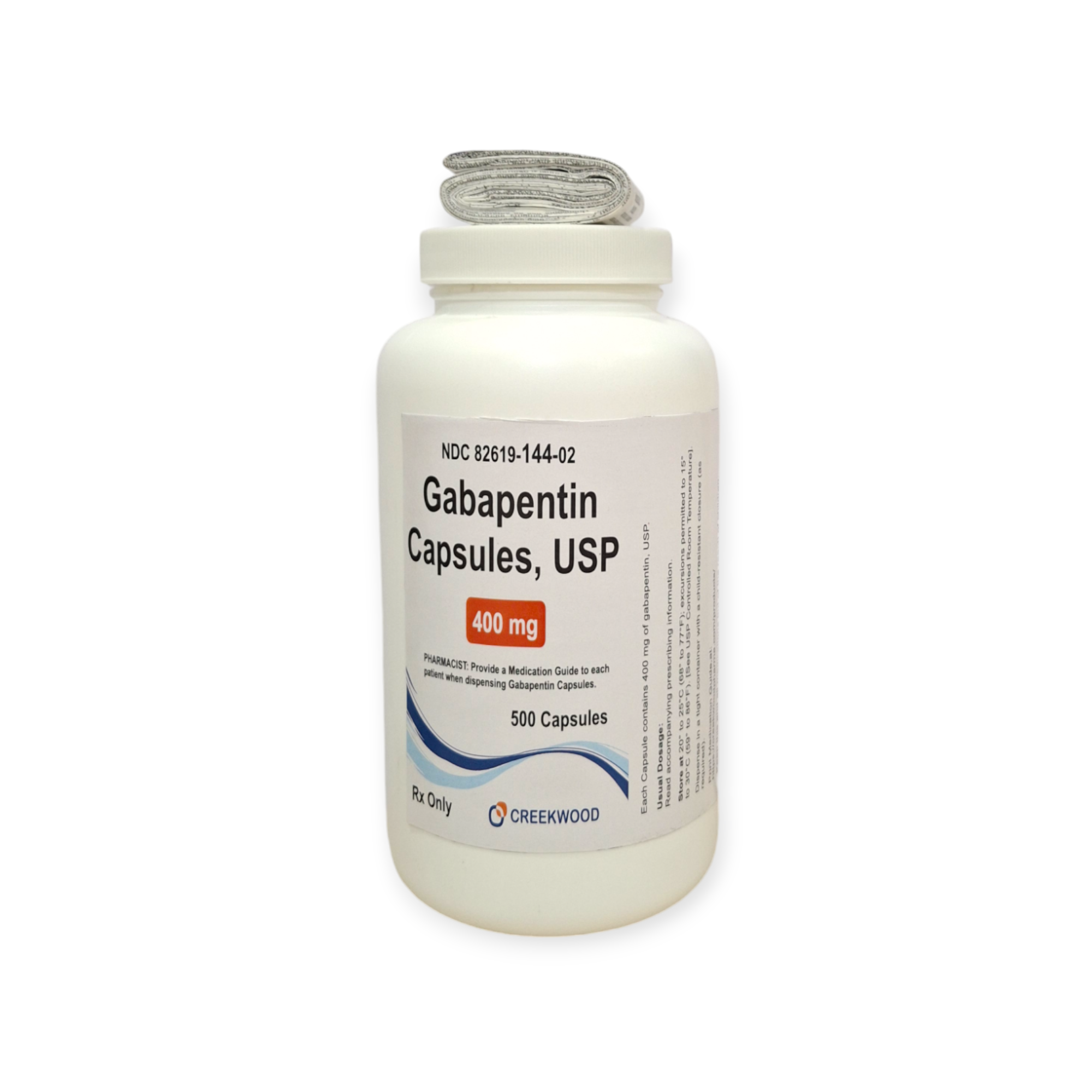
| NDC | 82619-144-02 |
| GTIN | 00382619144024 |
| Strength | 400 mg |
| Description of Pill | White to Off white powder filled in size “0” hard gelatin capsules with opaque Orange colored cap and opaque Orange colored body imprinted “SG” on cap and “181” on body with black ink. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 500 |
| Therapeutic Class | Anticonvulsant |
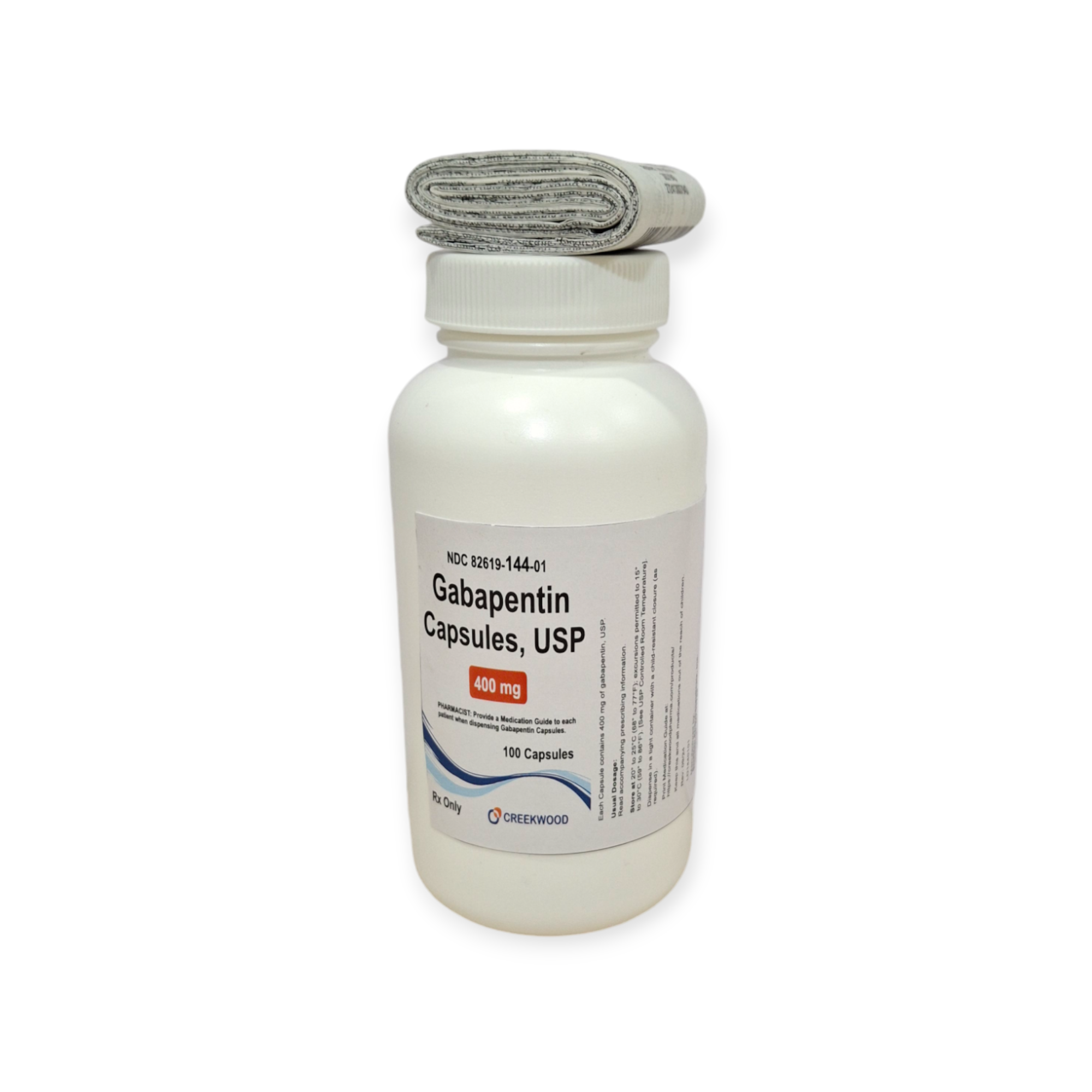
| NDC | 82619-144-01 |
| GTIN | 00382619144017 |
| Strength | 400 mg |
| Description of Pill | White to Off white powder filled in size “0” hard gelatin capsules with opaque Orange colored cap and opaque Orange colored body imprinted “SG” on cap and “181” on body with black ink. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 100 |
| Therapeutic Class | Anticonvulsant |

| NDC | 82619-143-03 |
| GTIN | '00382619143034 |
| Strength | 300 mg |
| Description of Pill | White to off white powder filled in size “1” hard gelatin capsules with opaque Yellow colored cap and opaque Yellow colored body imprinted “SG” on cap and “180” on body with black ink |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 1000 |
| Therapeutic Class | Anticonvulsant |
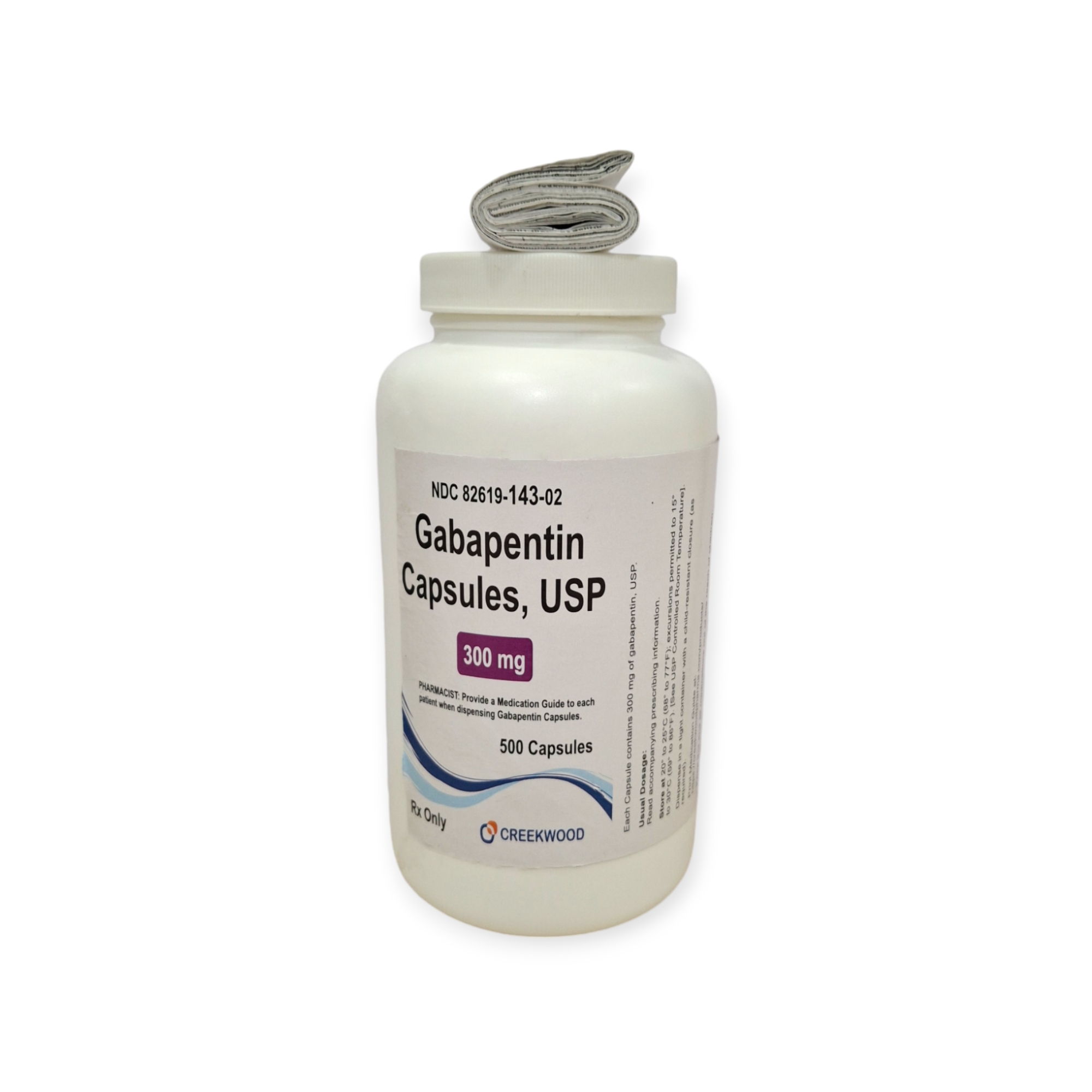
| NDC | 82619-143-02 |
| GTIN | 00382619143027 |
| Strength | 300 mg |
| Description of Pill | White to off white powder filled in size “1” hard gelatin capsules with opaque Yellow colored cap and opaque Yellow colored body imprinted “SG” on cap and “180” on body with black ink |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 500 |
| Therapeutic Class | Anticonvulsant |
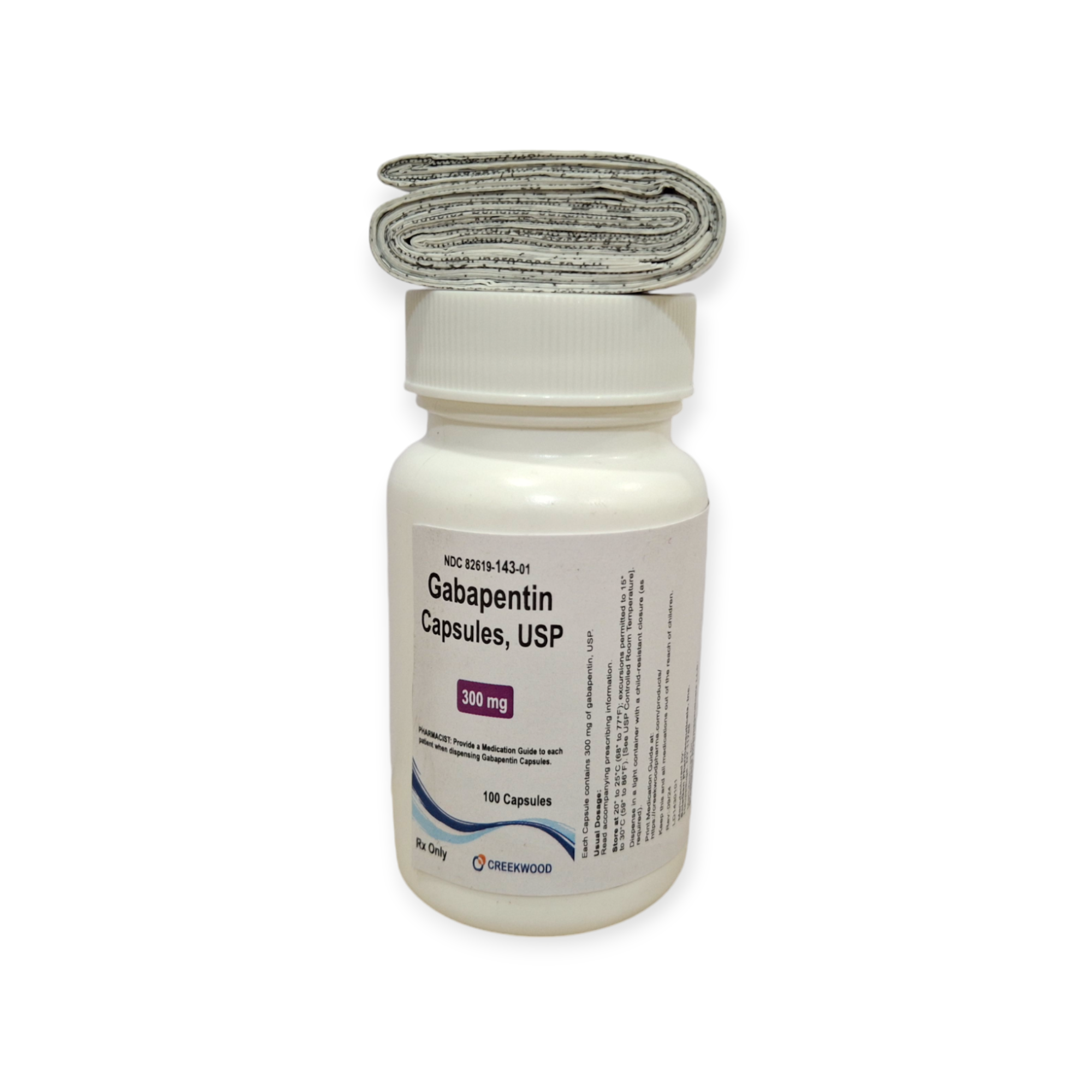
| NDC | 82619-143-01 |
| GTIN | '00382619143010 |
| Strength | 300 mg |
| Description of Pill | White to off white powder filled in size “1” hard gelatin capsules with opaque Yellow colored cap and opaque Yellow colored body imprinted “SG” on cap and “180” on body with black ink |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 100 |
| Therapeutic Class | Anticonvulsant |
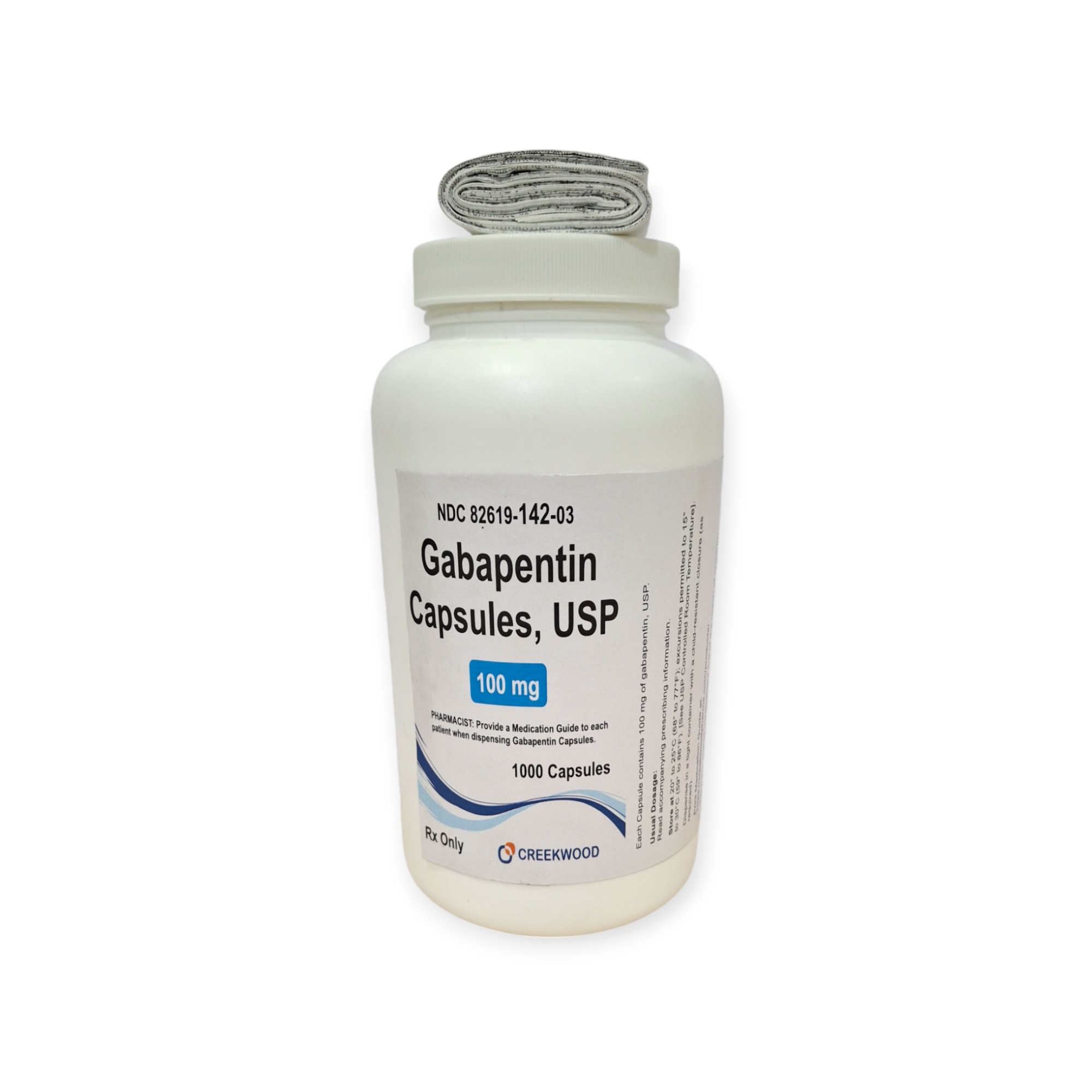
| NDC | 82619-142-03 |
| GTIN | 00382619142037 |
| Strength | 100 mg |
| Description of Pill | White to Off white powder filled in size “3” hard gelatin capsules with opaque White colored cap and opaque White colored body imprinted “SG” on cap and “179” on body with black ink |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 1000 |
| Therapeutic Class | Anticonvulsant |
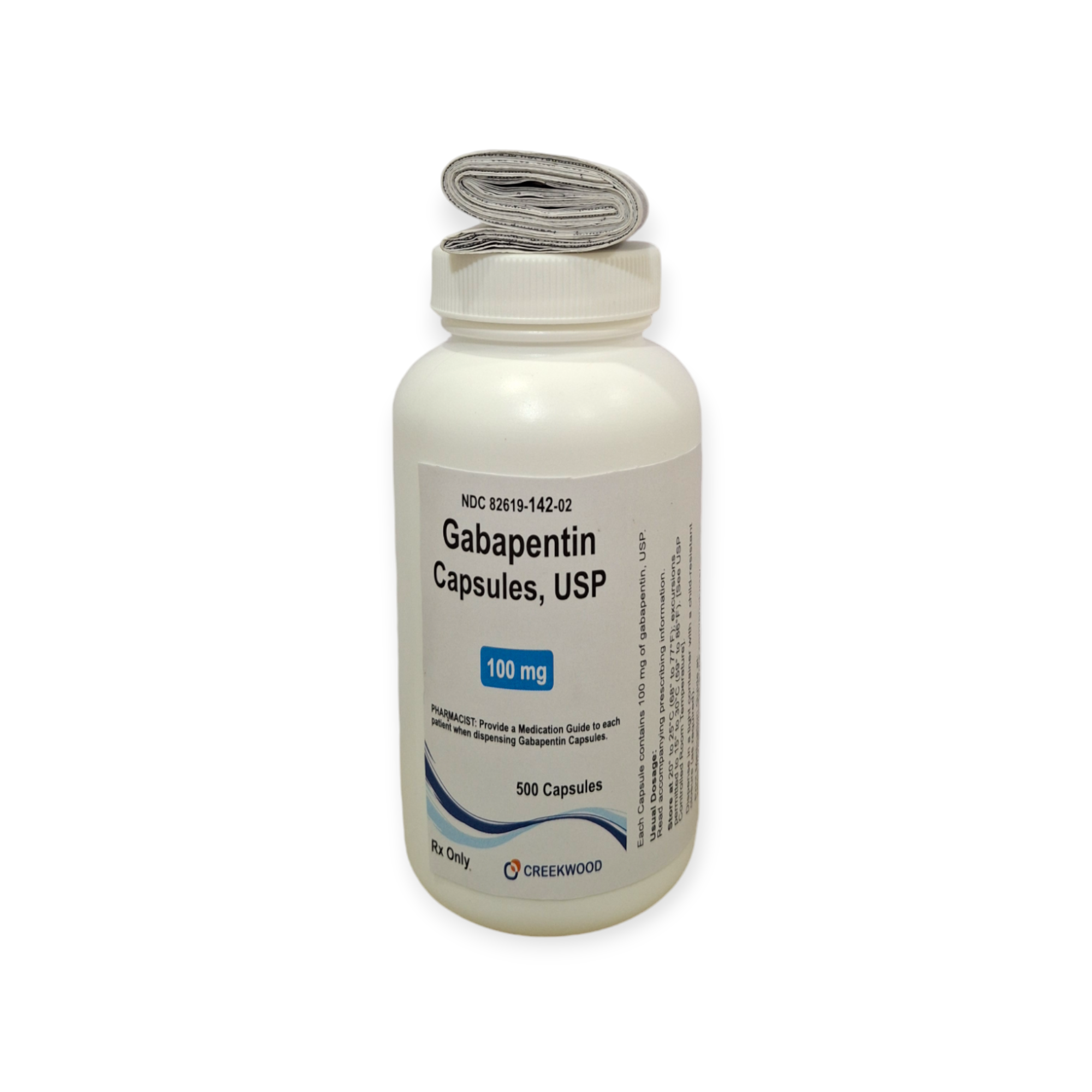
| NDC | 82619-142-02 |
| GTIN | 00382619142020 |
| Strength | 100 mg |
| Description of Pill | White to Off white powder filled in size “3” hard gelatin capsules with opaque White colored cap and opaque White colored body imprinted “SG” on cap and “179” on body with black ink |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 500 |
| Therapeutic Class | Anticonvulsant |
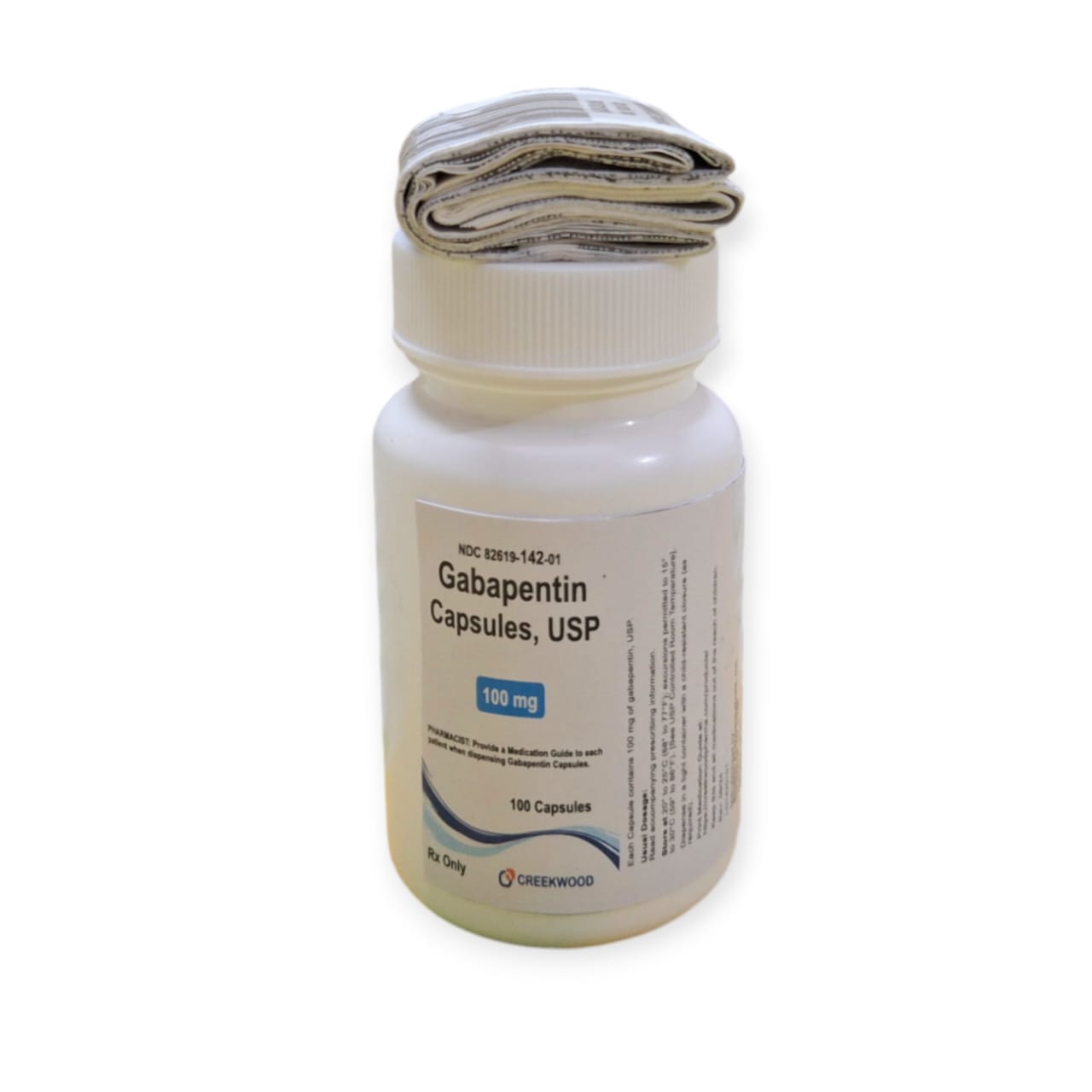
| NDC | 82619-142-01 |
| GTIN | 00382619142013 |
| Strength | 100 mg |
| Description of Pill | White to Off white powder filled in size “3” hard gelatin capsules with opaque White colored cap and opaque White colored body imprinted “SG” on cap and “179” on body with black ink |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Neurontin |
| Pack Size | 100 |
| Therapeutic Class | Anticonvulsant |

| NDC | 82619-141-01 |
| GTIN | 00382619141016 |
| Strength | 750 mg |
| Description of Pill | Metformin Hydrochloride Extended-Release Tablets, USP 750mg, 100 tablets per bottle. White to off white, oval (Modified Capsule) shaped tablet debossed with "M17" on one side and plain on another side. |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | MAXALTGLUCOPHAGE® XR |
| Pack Size | 100 |
| Therapeutic Class | Anti-hyperglycemic |

| NDC | 82619-140-02 |
| GTIN | 00382619140026 |
| Strength | 500 mg |
| Description of Pill | Metformin Hydrochloride Extended-Release Tablets, USP 500mg, 500 tablets per bottle White to off white, oval (Modified Capsule) shaped tablet debossed with "M16" on one side and plain on another side. |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB1 |
| Brand Reference | MAXALTGLUCOPHAGE® XR |
| Pack Size | 500 |
| Therapeutic Class | Anti-hyperglycemic |

| NDC | 82619-140-01 |
| GTIN | 00382619140019 |
| Strength | 500 mg |
| Description of Pill | Metformin Hydrochloride Extended-Release Tablets, USP 500mg, 100 tablets per bottle White to off white, oval (Modified Capsule) shaped tablet debossed with "M16" on one side and plain on another side. |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB1 |
| Brand Reference | MAXALTGLUCOPHAGE® XR |
| Pack Size | 100 |
| Therapeutic Class | Anti-hyperglycemic |

| NDC | 82619-112-05 |
| GTIN | 00382619112054 |
| Strength | 10 mg |
| Description of Pill | Rizatriptan Benzoate, USP 10 mg tablets are Pale pink colored, capsule shaped uncoated tablets debossed with ‘389’ on one side and plain on other side |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | MAXALT |
| Pack Size | 18 |
| Therapeutic Class | ANTIMIGIRAINE |

| NDC | 82619-111-05 |
| GTIN | 00382619111057 |
| Strength | 5 mg |
| Description of Pill | Rizatriptan Benzoate, USP 5 mg tablets are Pale pink colored, capsule shaped uncoated tablets debossed with ‘388’ on one side and plain on other side. |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | MAXALT |
| Pack Size | 18 |
| Therapeutic Class | ANTIMIGIRAINE |
| NDC | 82619-102-01 |
| GTIN | 00382619102017 |
| Strength | 120 mg |
| Description of Pill | Fenofibrate Tablets, USP 120 mg are white to off-white, oval shaped, uncoated tablets debossed with "294" on one side and plain on other side |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | FENOGLIDE |
| Pack Size | 90 |
| Therapeutic Class | Primary Hypercholesterolemia, Hypertriglyceridemia |
| NDC | 82619-101-01 |
| GTIN | 00382619101010 |
| Strength | 40 mg |
| Description of Pill | Fenofibrate Tablets, USP 40 mg are white to off white, biconvex, round shaped, uncoated tablets debossed with "293" on one side and plain on other side. |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | FENOGLIDE |
| Pack Size | 90 |
| Therapeutic Class | Primary Hypercholesterolemia, Hypertriglyceridemia |

| NDC | 82619-112-04 |
| GTIN | 00382619112047 |
| Strength | 10 mg |
| Description of Pill | Rizatriptan Benzoate, USP 10 mg tablets are Pale pink colored, capsule shaped uncoated tablets debossed with ‘389’ on one side and plain on other side. |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | MAXALT |
| Pack Size | 12 |
| Therapeutic Class | ANTIMIGIRAINE |

| NDC | 82619-111-04 |
| GTIN | 00382619111040 |
| Strength | 5 mg |
| Description of Pill | Rizatriptan Benzoate, USP 5 mg tablets are Pale pink colored, capsule shaped uncoated tablets debossed with ‘388’ on one side and plain on other side. |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | MAXALT |
| Pack Size | 12 |
| Therapeutic Class | ANTIMIGIRAINE |
| NDC | 82619-114-02 |
| GTIN | 00382619114027 |
| Strength | 8 mg |
| Description of Pill | Silodosin 8mg Capsules . Size 1 hard gelatin capsules, with white opaque cap and body. The cap is imprinted in black ink with "480". The body is imprinted in black ink with '8'. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Rapaflo |
| Pack Size | 90 |
| Therapeutic Class | Alpha‑1 adrenergic receptor antagonist |
| NDC | 82619-114-01 |
| GTIN | 00382619114010 |
| Strength | 8 mg |
| Description of Pill | Silodosin 8mg Capsules . Size 1 hard gelatin capsules, with white opaque cap and body. The cap is imprinted in black ink with "480". The body is imprinted in black ink with '8'. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Rapaflo |
| Pack Size | 30 |
| Therapeutic Class | Alpha‑1 adrenergic receptor antagonist |
| NDC | 82619-113-01 |
| GTIN | 00382619113013 |
| Strength | 4 mg |
| Description of Pill | Silodosin 4 mg Capsules . Size 3 hard gelatin capsules, with white opaque cap and body. The cap is imprinted in black ink with "479". The body is imprinted in black ink with '4'. |
| OTC/Rx | RX |
| Dosage Form | Capsule |
| TE Code | AB |
| Brand Reference | Rapaflo |
| Pack Size | 30 |
| Therapeutic Class | Alpha‑1 adrenergic receptor antagonist |

| NDC | 82619-104-01 |
| GTIN | 00382619103014 |
| Strength | 1.25 mg + 2.5 mg |
| Description of Pill | ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE TABLETS FULL STRENGTH |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | Covaryx |
| Pack Size | 100 |
| Therapeutic Class | Hormone |

| NDC | 82619-103-01 |
| GTIN | 00382619103014 |
| Strength | 0.625 mg + 1.25 mg |
| Description of Pill | ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE TABLETS HALF STRENGTH |
| OTC/Rx | RX |
| Dosage Form | Tablets |
| TE Code | AB |
| Brand Reference | Covaryx |
| Pack Size | 100 |
| Therapeutic Class | Hormone |